
Greyed-out rows indicate values based on estimation rather than experiment. Some other elements in the middle of the 4d and 5d rows have been omitted (Zr–Tc, Hf–Os) when their simple cations are too highly charged or of rather doubtful existence. Metallicity has been defined by the " stairstep line", so germanium and antimony have been included, but not astatine (whose simple cation is doubtful in any case). Hydrogen has been included as a benchmark, although it is not a metal. (In any case, the typical oxidation states for the most accessible seventh-period elements thorium and uranium are too high to allow a direct comparison.) In the first six periods this does not make a difference to the relative order, but in the seventh period it does, so the seventh-period elements have been excluded. However, not all sources give the same values: there are some differences between the precise values given by NIST and the CRC Handbook of Chemistry and Physics. It is mostly based on tables provided by NIST. The following list includes the metallic elements of the first six periods.
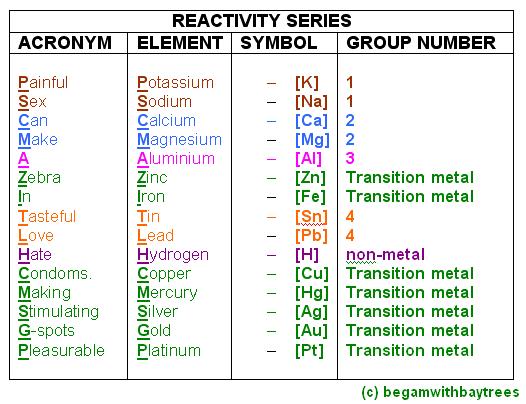
MEMORIZE ACTIVITY SERIES OF METALS SERIES
The reactivity series is sometimes quoted in the strict reverse order of standard electrode potentials, when it is also known as the " electrochemical series". Copper and silver will react with nitric acid but because nitric acid is an oxidizing acid, the oxidizing agent is not the H + ion as in normal acids, but the NO 3 − ion.Ĭomparison with standard electrode potentials
Magnesium, aluminium and zinc can react with water, but the reaction is usually very slow unless the metal samples are specially prepared to remove the surface layer of oxide which protects the rest of the metal. There is some ambiguity at the borderlines between the groups.

Metals in the middle of the reactivity series, such as iron, will react with acids such as sulfuric acid (but not water at normal temperatures) to give hydrogen and a metal salt, such as iron(II) sulfate:įe (s) + H 2SO 4 (l) → FeSO 4 (aq) + H 2 (g) The most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide:Ģ Na (s) + 2 H 2O (l) →2 NaOH (aq) + H 2 (g) There is no unique and fully consistent way to define the reactivity series, but it is common to use the three types of reaction listed below, many of which can be performed in a high-school laboratory (at least as demonstrations).

Reacts very slowly with cold water, but rapidly It is used to summarize information about the reactions of metals with acids and water, single displacement reactions and the extraction of metals from their ores. In chemistry, a reactivity series (or activity series) is an empirical, calculated, and structurally analytical progression of a series of metals, arranged by their "reactivity" from highest to lowest. In this article, the preparation, electronic properties and design of metal acyclic carbene complexes with different functional properties for the development of advanced materials are described.Not to be confused with Electrochemical series. The environmental sensitivity of the properties of these complexes also made them ideal for the development of stimuli-responsive materials and chemical sensors.

As a result, the functional properties of acyclic complexes can be drastically varied by rational molecular design of the ligands. Moreover, the open structure of acyclic carbene ligands has made their structure and the electronic properties strongly dependent on the substituents as well as the micro-environment. As transition-metal acyclic carbene complexes can be prepared from metal isocyanide synthetic precursors, substituents of different electronic and steric natures as well as functional moieties can be readily introduced into acyclic carbene ligands by changing the isocyanide ligand. Therefore, transition-metal acyclic carbene complexes are considered viable alternatives to NHC complexes in the development of metal-based functional materials. As acyclic carbene ligands show strong σ-donating properties comparable to N-heterocyclic carbene (NHC) ligands, transition-metal complexes with acyclic carbene ligands also demonstrate outstanding performance and functional properties similar to their NHC counterparts. Transition-metal acyclic carbene complexes have received increasing attention in recent years.


 0 kommentar(er)
0 kommentar(er)
