

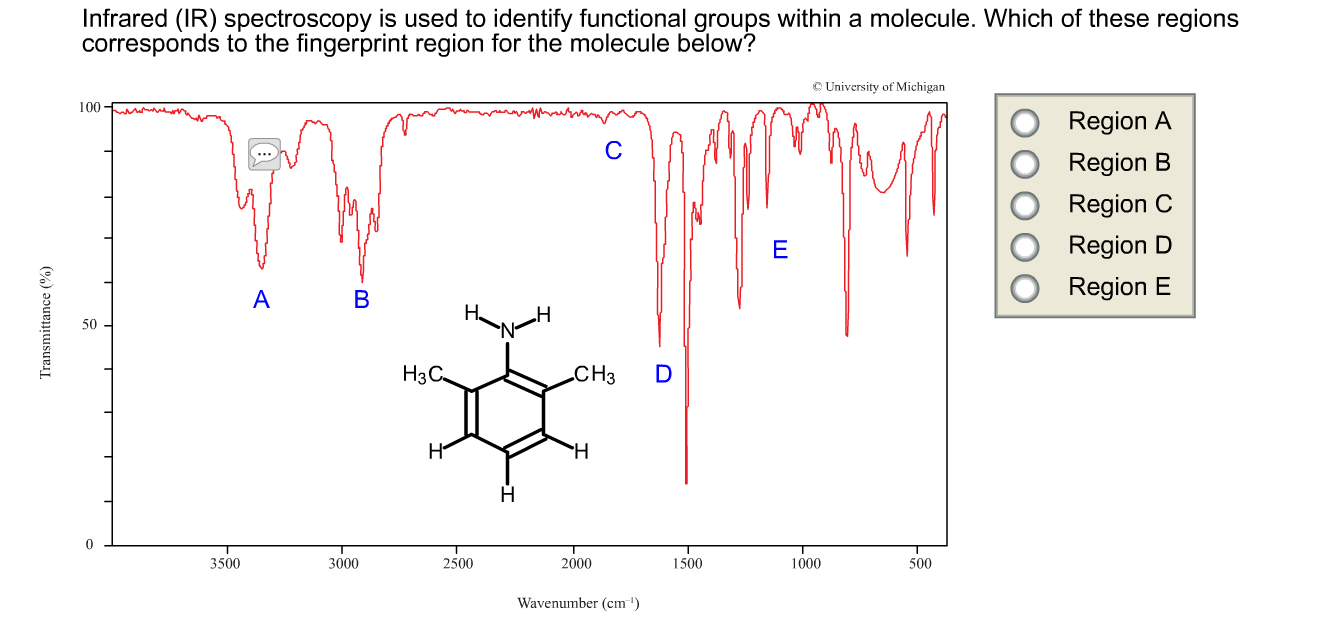
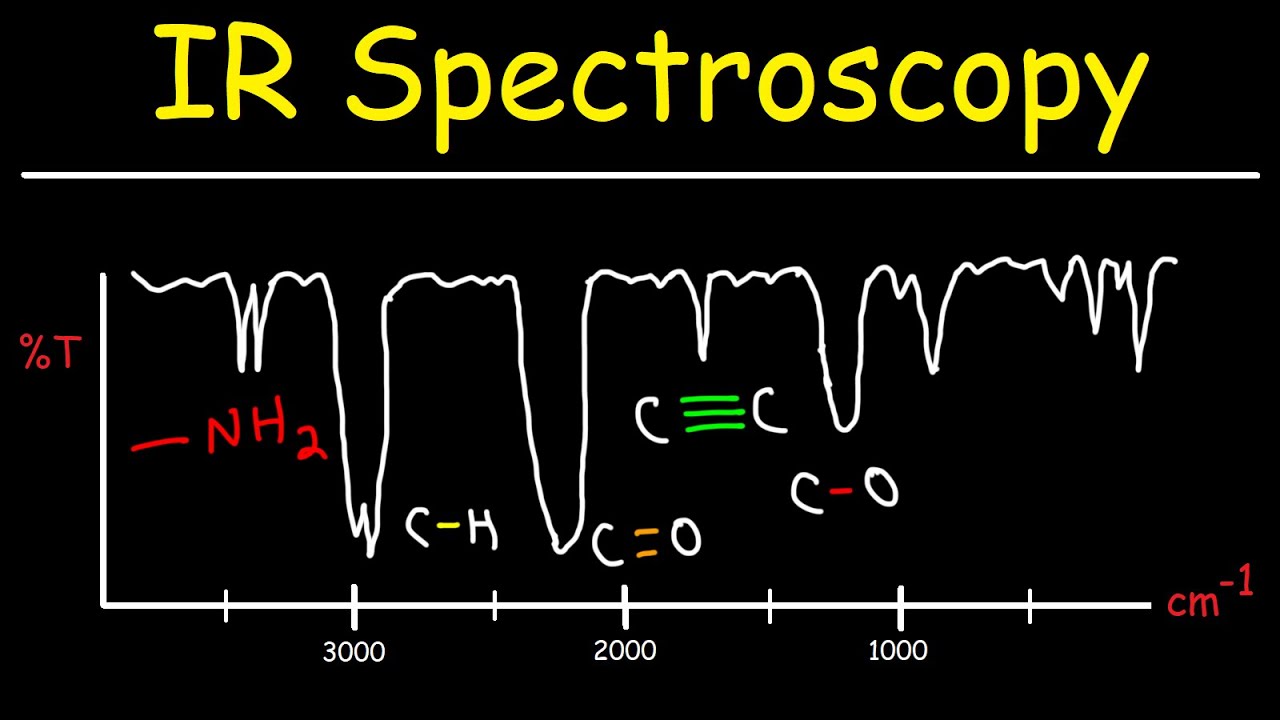
(y) the use of infrared spectroscopy to identify the presence of certain bonds in organic molecules thereby indicating whether they may be alkanes, alkenes, alcohols or carboxylic acids.2.5 CRUDE OIL, FUELS AND ORGANIC CHEMISTRY.Unit 2: CHEMICAL BONDING, APPLICATION OF CHEMICAL REACTIONS and ORGANIC CHEMISTRY.(f) use of high resolution ¹H NMR spectra (alongside the other spectral data specified in 2.8) in the elucidation of structure of organic molecules.(b) use of IR spectra in identification of chemical structure.Unit 2: ENERGY, RATE AND CHEMISTRY OF CARBON COMPOUNDS.Infrared absorbances are measured in wavenumbers, the reciprocal of wavelength, in units of cm⁻¹.In infrared spectroscopy, infrared radiation is passed through a sample of the organic compound and then into a detector which measures the intensity of the transmitted radiation at different wavelengths.There are two tables grouped by frequency range and compound class. The table lists IR spectroscopy frequency ranges, appearance of the vibration and absorptions for functional groups. The wavelengths which are absorbed to cause the vibrations (stretches and bends) will depend on the type of chemical bond and the groups or atoms at the ends of these bonds. The IR Spectrum Table is a chart for use during infrared spectroscopy.Infrared radiation causes parts of a molecule to vibrate.Infrared spectroscopy can be used to identify certain functional groups in an organic compound.Organic chemistry and instrumental analysis.a) infrared (IR) radiation causes covalent bonds to vibrate more and absorb energy IR spectrum chart Advantages, disadvantages and uses of mid vs near-IR/FTIR spectroscopy FTIR applications present and future What is IR spectroscopy The human eye can only see a small part of the much broader spectrum of electromagnetic radiation (Figure 1).Bonds in a molecule absorb infrared radiation at characteristic wavenumbers.Infra-red absorption spectrometry (IR) as a 'fingerprinting' technique involving absorption of infra-red radiation (reference to molecular vibrations not required).
Abstract Infrared (IR) spectroscopy is an absorption method widely used in both qualitative and quantitative analyses.
#Infrared spectroscopy chart pdf
The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
